

Smart dental implants (SDIs) are transforming the field of oral healthcare by integrating advanced materials, sensors, and energy harvesting technology. These innovative implants address key challenges like implant failure and peri-implantitis through antimicrobial materials, built-in phototherapy, and real-time health monitoring. By harnessing piezoelectric nanoparticles, SDIs generate electricity from natural oral motions such as chewing and brushing, eliminating the need for external power sources. This renewable energy powers LEDs for light therapy, which promotes healing and reduces inflammation. Additionally, embedded micro-sensors wirelessly transmit oral health data, allowing for early detection of potential issues. Recent research demonstrates the effectiveness of SDIs in reducing bacterial biofilm formation and enhancing implant durability, promising better outcomes for patients. As the global population ages and the demand for dental implants increases, SDIs represent a groundbreaking advancement with applications beyond dentistry, including joint replacements and other medical implants. While challenges remain in terms of cost, scalability, and biocompatibility, the future of SDIs is bright, offering a smarter, more sustainable approach to oral health.
Key words: Piezoelectric nanoparticles, Photobiomodulation therapy, Micro-sensors.
Dental implants are a widely used solution for
tooth replacement, with millions of procedures
performed globally each year.1 Despite their
success, traditional implants face significant
challenges, including peri-implantitis, bacterial
infections, and eventual failure.2 After tooth
extraction the remnants of the periodontal
ligament break down and disappear; and with
them the information on the force exerted when
biting and chewing is lost, as well. This lack of
information justifies the frequent failure and
breakage of dental prostheses These issues not
only impact patient outcomes but also increase
healthcare costs and the need for revision
surgeries.3
Accurate implant placement begins with precise
diagnosis and planning. While panoramic and
periapical images were once standard, CBCT
scans have become essential. They provide
3D imaging, cross-sectional views, and digital
DICOM data, which enable virtual planning,
creation of surgical guides, and prosthesis
fabrication before surgery.4 The increasing
adoption of dental implants for treating missing
teeth is driven by advancements in implant
dentistry and supported by growing research on
implant design, materials, and clinical behavior.
Although implant sales are rising worldwide, North America lags behind Europe, which led
the global market in 2016.5
The development of Smart Dental Implants
(SDIs) addresses these limitations by combining
advanced materials, energy-harvesting
technology, and embedded sensors to enhance
functionality and longevity.1 SDIs offer a
revolutionary approach to oral healthcare,
utilizing piezoelectric nanoparticles to generate
electricity from natural oral motions. This
renewable energy powers LEDs for phototherapy,
which aids in healing and infection prevention.2
Additionally, SDIs are equipped with micro
sensors that monitor oral health metrics such
as pH, temperature, and bacterial presence.
This data is transmitted wirelessly to healthcare
providers, enabling proactive intervention.25
This article explores the components,
applications, materials, challenges, and future
perspectives of SDIs, highlighting their potential
to redefine patient care.
The Smart Dental Implant (SDI) system utilizes a screw-retained crown design, a widely accepted
clinical standard. Its primary components
include an implant abutment, a dental crown,
integrated circuitry, micro LEDs, and a securing
screw. A distinguishing feature of the SDI is its
energy-harvesting dental crown, designed to
convert natural oral motions, such as chewing
and brushing, into electrical energy. This is
achieved through a carefully engineered two
phase composite structure:
Piezoelectric Material
The crown incorporates barium titanate
nanoparticles (BTNPs), a lead-free piezoelectric
material ideal for biomedical use. These
nanoparticles generate electrical energy in
response to mechanical stress, which powers the
implant’s functions.
Two-Phase Composite Design
0–3 Composite: Features 0-dimensional BTNPs
embedded in a 3-dimensional matrix, enhancing
the interaction between piezoelectric particles
and oral biomechanics for efficient energy
harvesting.
1–3 Composite: Combines 1-dimensional
resin pillars with a 3-dimensional BTNP-based
composite to provide the mechanical strength
needed to endure the forces from chewing and
brushing. (Fig.1)
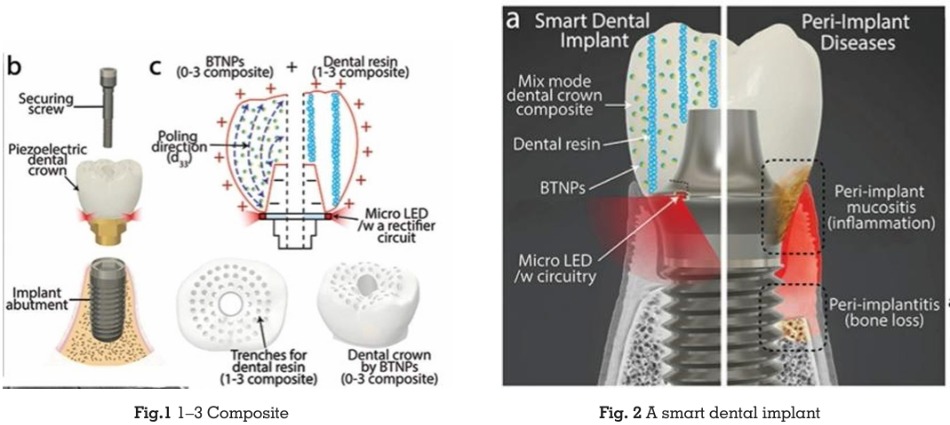
Customized Fabrication
The piezoelectric crown is manufactured using
paste extrusion 3D printing, a technique that
allows for the production of patient-specific
designs. This customization ensures the
crown fits the patient’s unique anatomy while
maintaining optimal functionality.1
Our study demonstrated that the SDI system
effectively harnesses human oral motion to
generate sufficient energy for preventing peri
implant disease. However, the performance
was tested under ideal conditions, such as
continuous oral motion at optimal frequencies.
To enhance functionality, a transistor switch
could be incorporated into the circuitry to store
harvested energy during oral motion and release it later when sufficient power is accumulated to
operate the LEDs.
Long-term reliability will require improved
packaging. While the current parylene coating
provides adequate moisture and dielectric
protection, more robust sealing is necessary
to fully isolate embedded electronics from
the oral environment. Embedding multiple
LEDs at the base of the crown could enhance
Photobiomodulation therapy by ensuring
comprehensive coverage of surrounding
gingival tissues, where peri-implant diseases
typically develop. Finally, while the current SDI
uses dental resin, which may not match the
mechanical strength of commercially available
dental crowns, exploring advanced materials
like zirconia could significantly improve
durability and structural integrity.1 (Fig.2)
Integrated Sensors
Micro-sensors embedded in SDIs monitor vital oral health metrics, including pH, temperature,
and bacterial presence. These sensors transmit
data wirelessly to healthcare providers, enabling
continuous monitoring and early intervention
(Fig. 3).24
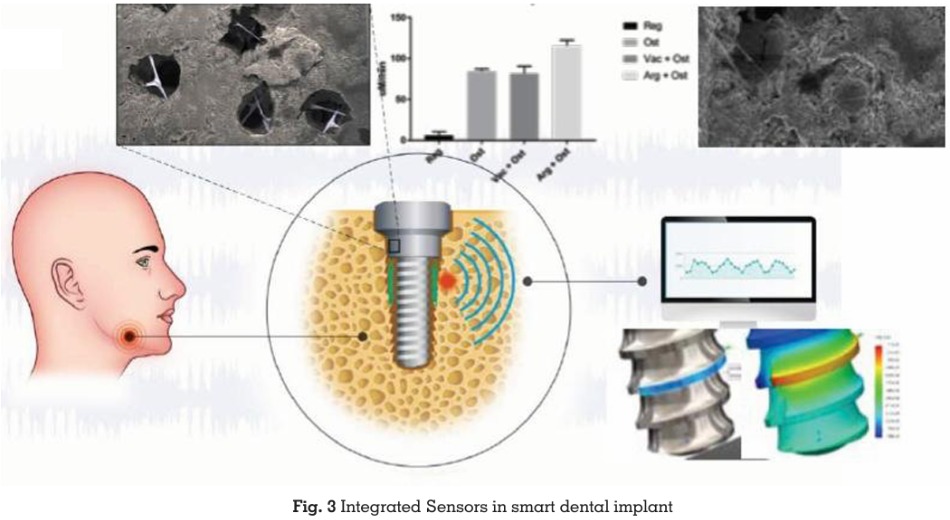
Applications
The primary application of SDIs is in oral
healthcare, where they address challenges such
as implant failure, infection, and inflammation.
By providing real-time monitoring and built-in
phototherapy, SDIs improve patient outcomes
and reduce the risk of complications.2
Beyond dentistry, the principles of SDIs have
broader applications in medicine. For example,
the same energy-harvesting and sensor
technologies could be applied to orthopedic
implants, such as joint replacements, to monitor
healing and detect early signs of failure.
Additionally, antimicrobial materials used in
SDIs could be adapted for use in other medical
devices, reducing infection risks and improving
patient safety.
Materials and Techniques
The materials and techniques behind
smart dental implants (SDIs) embody a
revolutionary integration of energy harvesting,
photobiomodulation therapy (PBMT),
antimicrobial defense, and real-time health
monitoring. These features address critical
challenges such as bacterial infections,
inflammation, and implant longevity. Central
to this innovation are advanced sensors,
piezoelectric materials, and a seamless
integration of therapeutic technologies.
Energy Harvesting through Oral Motions
A cornerstone of SDI technology is its ability
to convert mechanical energy generated by
natural oral activities chewing and brushing
into electrical energy. This is achieved through
the integration of piezoelectric nanoparticles, such as barium titanate nanoparticles (BTNPs).
These nanoparticles produce electrical charges
when subjected to mechanical stress, providing
a sustainable and self-sufficient energy source
for the implant.
Chewing Dynamics: Forces up to 200 N and
frequencies of 1–5 Hz generated during chewing
are converted into electrical energy.
Brushing Movements: The shear forces (15–70
N) and normal forces (12 N) associated with
brushing are harnessed to sustain implant
operations.
Energy Output: The harvested energy powers
critical implant functions, with light energy
densities reaching 0.77 μJ cm² s⁻¹ at 5 Hz,
equating to 4.1 mJ cm⁻² over 90 minutes of oral
activity.
This self-sustaining energy mechanism ensures
that the implant remains operational without
external power sources, eliminating the need for
battery replacements or recharging.1
Photobiomodulation Therapy (PBMT)
Powered by the harvested energy, PBMT is a key
feature of SDIs, utilizing embedded LED systems
to emit therapeutic wavelengths of light. Blue
light therapy is a clinically accepted approach
to kill a pathogen, such as Propionibacterium
acnes infections. This therapy offers multifaceted
benefits:
Bacterial Defense: The emitted light disrupts
bacterial biofilm formation, reducing the risk of
infections and implant failure.
Inflammation Reduction: PBMT mitigates
inflammation by modulating cellular activity in
the gum tissue, promoting faster healing and
reducing discomfort.
Tissue Regeneration: By stimulating collagen
production and cell proliferation, PBMT supports
the regeneration of gum tissue and enhances tissue integration with the implant.
The LEDs are strategically positioned within
the implant to ensure even distribution of
therapeutic light across the surrounding tissue,
maximizing the benefits of PBMT during routine
oral activities.1
Integrated Sensors for Real-Time Monitoring
A hallmark feature of SDIs is their embedded
micro-sensors, which continuously monitor
the implant’s surrounding environment. These
sensors are powered by the energy harvested
from oral motions and provide critical insights
into oral health parameters, including:
pH Levels: Variations in pH can indicate
bacterial activity or the onset of infections.
Temperature:
Monitoring temperature
fluctuations helps detect inflammatory responses
or potential complications.
Bacterial Activity: Real-time data on bacterial
colonization around the implant site enables
early intervention.24
The data collected by these sensors is wirelessly
transmitted to external devices such as
smartphones, tablets, or dental office systems.
This enables real-time monitoring by dental
professionals, allowing them to predict potential
failures, address issues early, and optimize
patient outcomes.25
Antimicrobial Coatings and Surface Design
To further enhance infection prevention, SDIs
incorporate antimicrobial coatings using BTNPs.
These coatings create a hostile environment
for bacterial adhesion and biofilm formation,
reinforcing the implant’s defenses.
Modeling and Simulating Oral Motions
To optimize the implant’s energy-harvesting
capabilities and durability, researchers utilize
advanced simulation techniques:
Chewing
Machines:
Electromechanical
universal test machines simulate chewing
forces and frequencies, ensuring the implant’s
performance under real-life conditions.
Brushing Apparatus: Custom rotational devices
replicate brushing movements, testing the
implant’s resilience and energy efficiency.1
Mechanical and Biomechanical Testing
Mechanical testing ensures the structural
integrity of SDIs. Flexural strength and modulus
are assessed using three-point bending tests on
composite materials infused with BTNPs. These
evaluations confirm the implant’s capacity to
endure the mechanical stresses of daily oral
activities.1
Biocompatibility and Cellular Studies
Biocompatibility is a critical factor for
SDIs. Cellular studies with human gingival
keratinocytes (HGKs) validate the safety and
efficacy of PBMT and antimicrobial coatings.
When exposed to bacterial lipopolysaccharides
(LPS), cells treated with PBMT show reduced
inflammation and improved
viability,
demonstrating the therapy’s protective and
regenerative properties.
Synergy Between Sensors and PBMT
The integration of sensors and PBMT creates a
synergistic approach to oral health management.
Sensors detect early signs of bacterial growth
or inflammation, while PBMT actively mitigates
these issues. This dual mechanism ensures a
proactive and comprehensive defense against
complications.
Innovative Material Science and Future
Enhancements
Emerging designs for SDIs include asymmetric
surfaces, with one side optimized for tissue
integration and the other for bacterial resistance.
This approach balances healing and infection prevention, enhancing the implant’s overall
performance.
Tooth loss is a significant life event that affects
two essential functions—eating and speaking—
and has considerable impacts on various
aspects of quality of life.6 Patients fitted with
conventional removable dentures reported low
satisfaction and only modest improvements in
quality of life compared to those rehabilitated
with implants.7 The outcome of oral treatment
with conventional dentures, whether successful
or not, depends on various factors, including
the practitioner’s technical expertise and
challenging oral conditions.8
Research highlights the critical role of
bone volume in planning oral implants,
recommending a minimum of 10 mm in height
and 6 mm in width for the maxilla, and 6 mm
in height and 5 mm in width for the mandible,
to ensure successful implantation.9 Periodontitis
and cigarette smoking are linked to a higher risk
of implant failure, as they reduce the vascularity
of local tissues and disrupt healing, chemotaxis,
and systemic immunity.10
The distinction between a failed implant and
a failing implant is clinically significant. A
failed implant is typically identified by a lack
of osseointegration, characterized by implant
mobility and peri-fixtural radiolucency. In
contrast, a failing implant refers to a gradual
and ongoing process, marked by progressive
marginal bone loss without significant mobility.11
Prospective and retrospective studies report
success rates ranging from 84.9% to 100% in
longitudinal studies spanning up to 24 years.
However, failures, though infrequent, often
occur unexpectedly. In addition to implant loss,
early marginal bone loss around endosseous
implants is also considered a sign of failure.
Implant loss is categorized as either early
failure, occurring before osseointegration, or late failure, occurring after the implant is subjected
to occlusal load.12 Currently, partially edentulous
individuals constitute the largest and growing
group of patients seeking rehabilitation with
oral implants. Most of these patients are middle
aged, typically between 40 and 50 years old,
when they receive implants. Given the increasing
life expectancy, it is likely that these patients
will require their implant-supported restorations
to function effectively for several decades.13 In
fixed implant-supported dentistry, biological
and technical complications are common. These
issues can negatively affect the functionality
and aesthetics of the prosthesis, even with high
clinical expertise and proper prosthetic design.14
Peri-implant diseases are classified as either
peri-implant mucositis or peri-implantitis, both
of which are considered infectious conditions.
Peri-implant mucositis is characterized by
soft tissue inflammation around a functioning
dental implant, along with bleeding on probing
(BOP). In contrast, peri-implantitis involves
the loss of supporting marginal bone beyond
normal bone remodeling. While peri-implant
mucositis is believed to be reversible, peri
implantitis is more challenging to reverse.15 At
the 1st European Workshop on Periodontology
in 1993, peri-implantitis was defined as an
inflammatory reaction accompanied by the loss
of supporting bone in the tissues surrounding a
functioning implant (Albrektsson & Isidor, 1994).
However, this definition lacked specific clinical
and radiological criteria for inflammation and
bone loss, which hindered detailed analysis
of the various risk factors contributing to peri
implantitis.16 The need to assess the prevalence
of peri-implant diseases at the subject level was
emphasized, highlighting that the prevalence of
mucositis is approximately 80% at the subject
level and around 50% at the implant level. Peri
implantitis was found to occur in 28% to over
56% of subjects and in 12% to 43% of implants
in the study.17
The transition from peri-implant mucositis to periimplantitis marks the onset of peri-implantitis.
However, assessing this shift is challenging, as
it requires identifying early signs of supporting
bone loss. Additionally, documenting the onset
of the disease from a research perspective
necessitates a longitudinal approach. While a
prospective study may not be ethically feasible,
a retrospective evaluation of peri-implant bone
loss in radiographs of patients with advanced
peri-implantitis is justifiable. In addition to
determining the onset of peri-implantitis,
radiographs can also be used to assess the
disease’s progression.18
Peri-implantitis was defined by the presence
of plaque, suppuration, bleeding on probing
(BOP), and probing depth (PD) greater than 5
mm. The analysis of peri-implant sulcular fluid
was also used as a diagnostic aid, though
no specific marker for peri-implantitis was
identified. Variations of these diagnostic criteria
for peri-implant diseases are also found in the
literature. For instance, peri-implant mucositis
was diagnosed based on BOP/suppuration and
a PD greater than 4 mm, while peri-implantitis
required a PD greater than 5 mm, along with
radiographic bone loss of more than 0.2 mm
annually or progressive bone loss exceeding
3 threads, combined with signs of peri-implant
mucositis.19 The peri-implant mucosal connective
tissue attachment shares some clinical and
histological similarities with that of natural teeth.
However, the key difference lies in the cellular
composition and fiber orientation. The connective
tissue around a dental Implant is in direct contact
with the titanium dioxide surface and contains a
dense network of collagen fibers. These fibers,
arranged in major bundles, originate from
the periosteum of the alveolar bone crest and
extend to the mucosal margin, running parallel
to the implant/abutment surface. In contrast,
the connective tissue attachment teeth involves
collagen fibers that insert perpendicularly into
the root cementum.20
The difference in the orientation of gingival fibers around implants, compared to natural
teeth, is a key finding related to peri-implant
mucosa. This variation allows bacteria to more
easily penetrate the epithelial layer and reach
the connective tissue, contributing to increased
breakdown of soft tissues around implants.21
Bacterial infections are the primary cause
of dental implant failure. The bacterial flora
associated with periodontitis and peri-implantitis
are found to be similar. The microorganisms
most commonly linked to implant failure include
Gram-negative anaerobes such as Prevotella
intermedia, Porphyromonas
gingivalis,
Aggregatibacter actinomycetemcomitans,
Bacteroides forsythus, Treponema denticola,
Prevotella nigrescens, Peptostreptococcus
micros, and Fusobacterium nucleatum.22
Cell-to-cell contact in a physiological context can
occur between the same type of cells or between
different cell types, such as keratinocytes and
fibroblasts, which form the soft tissue seal
around dental implants.23
Photobiomodulation (PBM) therapy, also called
low-level light therapy (LLLT), has gained
attention for its significant biological benefits.
It effectively promotes tissue healing, reduces
inflammation, and mitigates bacterial activity,
making it a promising approach in addressing
peri-implant complications. Our SDI system
is an enhanced version of traditional dental
implants, featuring energy harvesting and light
delivery through a piezoelectric dental crown
and integrated LEDs. Mechanical actions such
as chewing and brushing generate electrical
energy, which is stored in a capacitor and then
used to power the LEDs.1
Challenges
While SDIs offer numerous benefits, they also
face significant challenges. These include the
high cost of materials and manufacturing,
ensuring long-term biocompatibility, and
navigating complex regulatory pathways for
approval. Additionally, integrating multiple technologies into a single implant requires
precise engineering and robust testing to ensure
reliability.
Future Perspectives
As research progresses, SDIs are expected
to become more cost-effective and widely
available. Advances in materials science and
manufacturing techniques will further enhance
their functionality and durability. Moreover, the
principles of SDIs could be extended to other
medical applications, revolutionizing healthcare
across multiple fields.
Smart dental implants represent a
groundbreaking advancement
in oral
healthcare, addressing key challenges like
infection, inflammation, and implant failure. By
integrating energy-harvesting nanoparticles,
antimicrobial materials,
and real-time
monitoring capabilities, SDIs offer a smarter,
more sustainable solution. While challenges
remain, the future of SDIs is promising, with the
potential to transform implant technology and
improve patient outcomes worldwide.