

Digitalised prosthetic rehabilitation modalities are becoming an essential approach for maxillofacial prosthetic field in present era. As technologies like nanotechnology, biotechnology, informatics, and cognitivism improve, maxillofacial amplification prostheses can now operate instinctively rather than passively. Through hybridization and the establishment of a genuine neurophysiological link with the environment, these can make maxillofacial amplification prostheses a reality. This narrative review emphasizes numerous technological advances in the field of maxillofacial rehabilitation, which, by resolving graft rejection and minimizing donor site morbidity, may pave the way for new avenues in reconstructive surgery.
Key words: Maxillofacial rehabilitation, 3D printing, trends, technologies
Maxillofacial deformity can result from malignancy, developmental trauma, or genetic flaws
that impair function and appearance, making
it difficult to lead a regular social life. Rehabilitating patients with craniofacial deformities has
historically been a difficult task due to the extended procedures and multiple clinical visits
needed for the fabrication of maxillofacial prostheses. People have been able to get beyond
these restrictions to some extent because to the
usage of cutting-edge materials and technology1-3. The industry has undergone a revolution in
digital technology, which have made the process
of designing, producing, assessing, and visualizing prostheses more efficient. The convergence
of technologies such as nanotechnology, biotechnology, informatics, and cognitivism (NBIC)
has made it possible for prostheses to introduce
new extrasensory capabilities and facilitate
authentic neurophysiological interactions with
their users. The biotechnological future of maxillofacial rehabilitation incorporating augmented reality with bioprinting along with 3D scanning
can make a patient experience new level of reality and comfort.
Maxillofacial prosthetics (MP) has long been
driven primarily by its strong association with
maxillofacial surgery. The field of maxillofacial
rehabilitation has historically been directed
by surgical considerations, and maxillofacial
prosthesis is a dependable and complementing option that fills in the gaps and resolves the
shortcomings that surgery alone cannot1. However, the field of maxillofacial rehabilitation is
changing as a result of the rapid development of
new technologies. This evolution is progressing
from simple repair to more advanced methods
that prioritize regeneration. As these technologies advance, they have the potential to improve
treatment outcomes and better satisfy patients
demands for all-encompassing, conclusive solutions to their maxillofacial problems2
Combining the terms cybernetic and organism,
neurophysiologist Manfred Clynes coined the
term “cyborg” in 1960 to denote a person who enhances or benefits from artificial means in their
biological functioning and was reintroduced
into the scientific literature following the work of
Donna Harway in its cyborg manifesto4
. These
techniques modifies the body biochemically or
electronically. Jean Claude Heudin proposed an
extensive classification of cybernetic phenotypes
from robots to avatars5
. Robotic and biological
cybornetic organisms are the two subcategories of cyborgs. Robotic Cybornetic organisms
are groups of organic molecules (e.g., the Terminator) on artificial structures that eventually
become humanoids which exist in science fiction
only. Conversely, those with sophisticated prostheses (robocops) are considered biological cyborgs and are already a part of our environment.
AR finds application in managing visual impairments like low vision, color vision deficiencies,
blindness, and visual field defects (Amblyopia,
Nyctalopia, and Metamorphopsia) HMD-based
AR systems and smartphone-based AR systems
are the two main types of AR prototypes. HMD based augmented reality systems comprise both
home-built and commercially produced AR systems, such as those made by Google and Microsoft. Google Glass is a wearable computing
device with an optical head-mounted projection
which works by combining both augmented and
virtual reality. Google introduced it in April 2012,
and the Google X lab developed to work based
on the Android operating system6.
Google Glass is built in with tiny chips that house a speaker, battery, video display, and camera. It has an Android-powered hands-free display and can establish Wi-Fi and Bluetooth connections with a phone. To record pictures and scenes that are within the wearer’s field of vision, tiny camera chips are used. On the video display, information is provided in a pop-up manner for hands-free viewing.
One of the major concerns of Google Glasses
is the possibility of privacy violations regarding
the user. Being expensive, only surgeons, military, astronauts, and elite athletes can use it.
A British start up, Place Ltd® unveiled the MindRDR® device in 2014, merging a control system
with Google glasses®. This technology detected
brain waves and converted them into commands
for augmented reality using a Neurosky® electro
encephalography biosensor placed on the user’s forehead7. A patent application for electronic contact lenses that display augmented reality
was also made by Google® in 2016. Although
this new device uses nanotechnology to fit inside
the polyethylene terephthalate lens,it can be
used for medical application by examining the
fluids on the cornea’s surface7-8.
The human nose is far more complex than the
ear or the sight, especially when it comes to the
systems that initiate the initial reaction to an
external stimuli. On the other hand, hundreds
of different types of biological receptors are involved in the sense of smell. Electronic noses
have made many interesting advances, but they
still don’t perform as well as our sense of smell
does. Artificial olfaction, utilizing “electronic
noses” consisting of three major components: a
sample handler, multiple gas sensors, and a signal processing technique.
An electronic nose is a machine that is designed
to detect and discriminate among complex
odours using a sensor array.
By making the sound audible, hearing aids
serve to treat hearing loss. The American Society Sonitus Medical® researchers came up with
the idea to use a detachable experimental prosthetic device called Soundbite® 11, mounted at
the level of the dental organs, to transmit sounds
through bone conduction in cases where the patient has a healthy inner ear but abnormalities
of the external auditory duct and/or tympanic
membrane. The receiver is placed on the ear, on
a pair of glasses, or on a jacket pin in order to
record ambient noises.
The SoundBite hearing system is an intraoral device created by Sonitus Medical12. The SoundBite hearing system works on bone conduction, it
may produce sound without the need for a working middle or outer ear. Bypassing the middle
and outer ears completely, the SoundBite hearing device is made to enable sound to pass via
the teeth, bones, and cochleae. The SoundBite is
designed to help people with SSD, conductive,
or mixed hearing loss regain normal hearing
without the need for surgery by employing bone
conduction via the teeth.
The SoundBite hearing system consists of a discrete, detachable in-the-mouth (ITM) device and
a behind-the-ear (BTE) microphone unit that
houses the receiver, wireless transmitter, and attached microphone. The tiny microphone is put
in the affected ear canal, where it is fitted with
an open dome to pick up noises. The SoundBite
hearing device is designed to take advantage
of the patient’s own pinna, or outer ear, which
naturally possesses the ability to capture and
guide sound by placing the microphone in the
ear canal. Following microphone capture, sound
is processed by the BTE digital audio device and
wirelessly sent to the detachable ITM hearing
aid. Through the use of cutting-edge technology,
the ITM gadget produces subtle sound vibrations
that go through the teeth, bone and cochlea.
An innovative low-frequency gadget known as an
audio implant12 was created in 2002, but it was
not removable, the process involved implanting
a sensor to detect noises in the inner ear through
bone conduction in a prosthetic tooth. One advantage was that voices seemed crystal like due
to the ability to detect vibrations below the average apparent frequency.
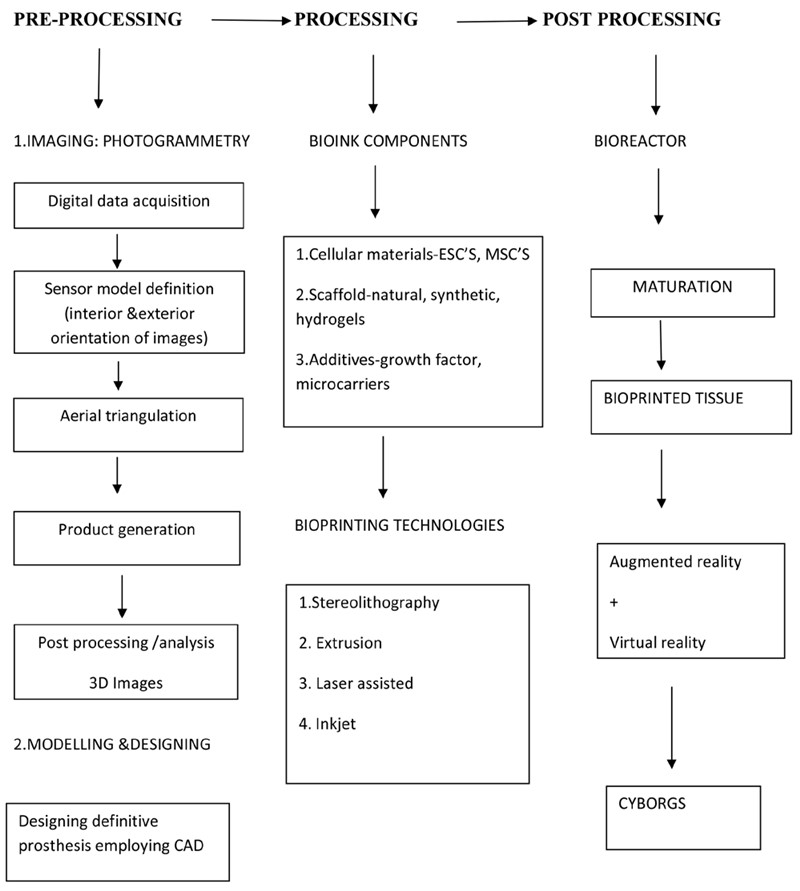
3D bioprinting is the process of printing biomaterials, bioactive factors, and even cells with precise placement and spatial control to recreate
human tissues and organs that closely resemble
their natural counterparts in terms of both structure and function. The technique is based on the
additive manufacturing which is combination of
tissue engineering and 3D printing15. One area
of regenerative medicine called tissue engineering uses patient cells to make autologous grafts.
Murphy and Atala described 3D bioprinting as,
‘‘layer-by-layer precise positioning of biological
materials, biochemicals and living cells, with
spatial control of the placement of functional components (extracellular matrix, cells and
pre-organized microvessels) to fabricate 3D
structures.’’16
A bioink is an integration of either differentiated
cells or stem cells and fluidic biomaterial17. It is
comparable to the cell-containing extracellular
matrix, which forms the scaffold when correctly
deposited and polymerizes or cross-links. As the
technology has advanced, it is now possible to
deposit many components of bioinks with exceptional accuracy, simulating the intricate architecture of human tissues, when previously only
a single bioink could be deposited. The specific
application, the kind of cells, and the bioprinter
to be utilized all influence the choice of bioink.
The current state of bioprinting technology is
insufficiently advanced to draw in the funding
required for proper development and to proceed to meaningful clinical trials. Since the inks
utilized in the technique did not contain any organic components, despite the fact that various
medicinal applications have been described,
they more closely align with the concept of 3D
printing than bioprinting. For instance, implants
used in cranioplasty20 are made especially to
address defects in the bone. The protocol states
that the patient’s tomographic data must be
used to determine the implant form and cutting
guide. A synthetic bone structure created in vitro is then used to fill the patient deficiency. This
procedure has been carried out using printed
hydroxyapatite, polyetherketoneketone (PEKK),
or polycaprolactone (PCL) inks21. Research on
oral bone-mucosa composites for palatal defect
reconstruction has also been done22.Within the
field of maxillofacial reconstruction, the same
3D printing technique proved beneficial for ear
and nose reconstruction23. Instead of using cartilage, printed acrylonitrile/butadiene/styrene
(ABS) scaffolds coated with hydrogel and chondrocytes or secondary coated with fibronectin
for biocompatibility were used to replace the
cartilage. Another group even printed an ear
that could hear noises that a typical human ear
cannot by seeding alginate hydrogel with chondrocytes and combining it with a conductive
electronic antenna24. The nanoelectronic components’ integrated silver nanoparticles allowed
the signals from the cochlea-shaped electrodes
to be read out. This proof-of-concept ear showed enhanced radio frequency reception auditory
perception as well as stereo audio perception.
The procedure utilized to extricate 3D data from
2D objects is called photogrammetry. The data is
procured by taking pictures of target spots that
reflect light, and after that utilizing those photographs to construct a three-dimensional model25.
Utilizing the suitable facilitate frameworks, the
common geometric relationship between the focuses and the picture is computed. This strategy includes taking all of the photographs with
the versatile gadget from different statures and
points, at that point nourishing the data into program to create a 3D model26. Since the mid19th
century, photogrammetry has been utilized for
3D photography, which was developed from radar, polygonal and radiometry. Photogrammetry
enables “Structure from Motion” (SFM), where
software examines the common characteristics
of each image describes and can build a 3D image from overlapping features using a complex
algorithm that minimizes the sum of errors in the
relative displacements of coordinates and reference points. This minimization is called “beam
regularization” and is often performed using the
Levenberg-Marquardt algorithm27.
Collection of Data
The new biotechnological methods promises
a vast array of potential maxillofacial applications, but their entrace on the healthcare market
is still undefined. The human organs are made
up of different cell types, matrices, and complex
configurations within each organ. Currently no
method can produce an entire organ or tissue
because of limitations in biomaterial compatibility, vascularity, resolution, and no defined
regulatory framework for bioprinted constructs.
As technology progresses in the realm of printing, and as more efficient and affordable printing techniques emerge, it’s essential to establish
and maintain quality control standards at every
stage of the process, including during model design, choosing the bioink, verifying the printing,
allowing the bioink to mature after printing, and
evaluating the quality of the final product.
Bioprinting involves a series of steps, each of
which must be carefully coordinated with the
others. Perfusion bioreactors are anticipated to
play a crucial role in the further integration of bioprinting technologies. However, beyond these
future prospects, the most critical aspect will be
the incorporation of bioinks with enhanced bioprintability and biofunctional characteristics.
Currently, the majority of bio-based materials employed in bioprinting are derived from polymers typically used in tissue engineering, and
they often lack the necessary rheological and
crosslinking properties that are essential for a
successful bioprinting process29. Moreover, given that the primary goal of bioprinting is to create
functional tissue constructs, there is also a need
for the development of more advanced assays
capable of evaluating cell functionality within
3D structures. Given the rapid advancement of
bioprinting technology and the widespread interest in this field across various scientific disciplines, it is anticipated that these challenges
can be addressed, leading to the availability of
bioprinted constructs for translational research
and accelerating the drug development process.
Cyborgology modifies maxillofacial prosthesis,
altering the wearer’s body depiction and self-image, resulting in new body sensations and perceptions of their inner selves and surroundings1
.
More research is required to combine bioprinted organic materials with artificial structures to
create a robotic, living entity. In the near future,
beneficiaries of this multidisciplinary approach
may be able to receive transformed tissues and
organs along with better neurophysiological
links to their surroundings. The advancement
of maxillofacial prosthetics for the future needs
constant ethical oversight3
. Unfortunately, the
scientists and engineers tasked with developing
tomorrow’s biotechnologies may not completely
comprehend the implications of their creations
for human evolutionary futures. This goes beyond simply replacing parts; it also includes
restoring sensory capacities, which improve the
brain’s ability to comprehend information.
Digital facial impressions using mobile device
photos enabled monoscopic photogrammetry to
generate 3D models. A less expensive option to
record the facial anatomy of patients using inexpensive free software. This would allow for the creation of physical working models, templates
for facial prostheses, improved patient communication before and during treatment, and increased access to digital clinical solutions for
clinical centers with limited technological resources. Standardizing a photo capture strategy28 for data capture and processing is crucial
since prolonged capture times with multiple images are prone to errors. The capture-to-print
prototype process will be made simpler with a
common photo capture technique.
In the fields of biotechnology and 3D scanning,
extensive research is being conducted which
promises, better future by overcoming challenges. While many of these technologies are in the
developing state, this integrated approach can
revolutionize future maxillofacial rehabilitation.
This review has highlighted the role of various
digital and biotechnologies in overseas maxillofacial prosthetic collaboration as an alternative to the conventional techniques. However,
introduction of new technologies and techniques
would require changes to current treatment protocols, workflow setting and training requirements. These challenges can be broadly considered as technological limitations and expenses.
Advancement in technology has a profound impact on the maxillofacial restoration of form and
function. However, creating indistinguishable
maxillofacial prostheses continues to be a challenge.