

Aim - The present investigation was done with
an aim to evaluate and compare the effect of two
chromatogens on color stability of three provisional
materials before and after thermocycling.
Materials and methods - Three commercially
available provisional materials were chosen -
DPI heat cure, Protemp™4 chemical cure and
the relatively newer Luxatemp Solar dual cure
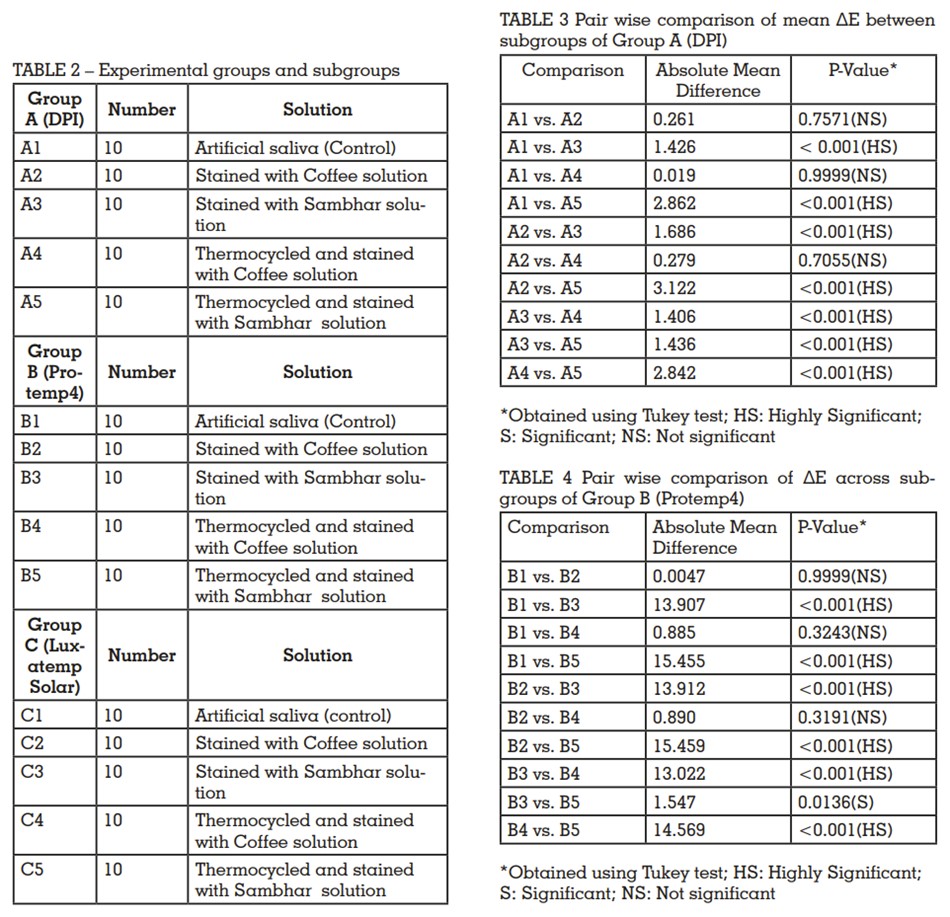
material. Flat circular metallic dies were prepared
of 22 mm diameter and 2 mm thickness. Total 150
samples were prepared from the materials using
these dies. The samples were finished and polished
using standardized methods. They were divided
into five groups. Two groups from each material
were subjected to a standardized thermocycling
regimen. The samples were immersed in two
staining solutions - coffee solution and sambhar
curry solution for 30 days. Artificial saliva was used
for the control group. The solutions were prepared
using a standardized method and were changed
every day. The color measurements were done twice
- once before thermocycling and staining and once
after, in CIE L*a*b* color system using a reflectance
spectrophotometer.
Results – Statistical analysis was done in SPSS version 20.0 using One-way ANOVA and Tukey’s
post-hoc test. p value <0.05 was considered
statistically significant.
Conclusions – New material Luxatemp Solar showed
least color stability, followed by Protemp™4 whereas
DPI showed maximum color stability. Sambhar curry
showed higher staining ability. The color changes
seen with coffee and control were not clinically
perceptible. Thermocycled samples showed more
color change than non thermocycled samples.
Key words: Color stability, provisional materials, coffee solution, sambhar curry solution, thermocycling.
CLINICAL IMPLICATIONS - DPI provisional restorative material showed better color stability than Protemp™4 and Luxatemp Solar. However, the values of color change were clinically acceptable with the control and coffee group for all materials. Hence, patients with a simple or a health conscious diet, or the ones regularly consuming coffee, can be given prosthesis using any provisional material. If the diet of the patient includes typical Indian curries, it is advisable to use DPI heat cure provisional material as it showed clinically acceptable color change with sambhar, which was the representative of Indian curries
Provisional restorations are an essential part
of fixed prosthodontic treatment.1 They are
designed to enhance esthetics, stabilization
and/or function for a limited period of time, after
which they are to be replaced by a definitive
prosthesis.2 These may be required to be placed
in the patients’ mouth for a few days to few
weeks. Occasionally, interim treatment has to
function for extended intervals and provide
long-term tooth protection and stability while
adjunctive treatment is accomplished,3 like in the
midst of the Covid pandemic where most dental
clinics performed only emergency treatments
globally or other emergency situations where
patient cannot report to the clinic. Amongst all its
functions, esthetics of the provisional restoration
is of prime importance especially in cases where
the provisional restorations are going to be used
for a long period of time and or are in the esthetic
zone.4 Discoloration of provisional restorations
may result in patient dissatisfaction and an
additional expense for their replacement5
,
adding to the number of visits and costs.
Regardless of their chemistry, most provisional
restorative materials are subject to sorption,
a process of absorption and adsorption of
liquids. As a result, color changes may occur
over time when these provisional restorations
are subjected to various staining agents.6
Indian food consists of various chromatogenic
substances such as tea, coffee, colas, turmeric
powder, red chilli powder, spices, oil, curry etc.
which are consumed on a daily basis and can
adversely affect the color of the provisional
restorative material.7
With globalization, Indian
food is now relished on a regular basis in most
parts of the world. A number of studies have
investigated the color stability of provisional
materials in various chromatogens. However, the
effect of commonly consumed chromatogens by
the Indian population on provisional restorative
materials has not been studied much.
Intraorally, temperature changes are seen
induced by routine eating and drinking.
Thermocycling can be done to simulate this
clinical situation in the laboratory.8 However,
limited data is available about the color stability
of provisional resins on temperature changes
subjected to thermocycling.
Today, with increased dental awareness
amongst patients and their improved standard
of living, it is imperative for the Prosthodontist
to provide a prosthesis which not only functions
efficiently, but also maintains its appearance
over the entire period of service.
Considering these facts, the present study
was undertaken to evaluate the color changes
that occurred when DPI heat cure, Protemp™4
chemical cure and the relatively newer
Luxatemp Solar dual cure provisional restorative
materials, were subjected to immersion in
coffee and sambhar curry solution, which are
common Indian chromatogens, for a period
of 30 days, before and after thermocycling,
which represented temperature changes
(Table1). The null hypothesis was that there is
no significant difference in the color stability of
tested provisional materials when exposed to
chromatogens or thermocycling.
The details of all materials are given in Table 1. The methodology was divided as follows:
ΔE= [(ΔL)2 + (Δa)2 + (Δb)2]1/2
Values ΔE > 3.7 was considered as clinically not
acceptable.21
The data of the color measurements was
obtained and subjected to statistical analysis.
The analyses were performed using SPSS version
20.0 (SPSS Inc.). The comparison of mean color
stability across groups was performed using
one-way analysis of variance (ANOVA). The pair
wise analysis was performed using Tukey’s post-hoc test. The p value <0.05 was considered as
statistically significant while p value <0.001 was
considered as highly significant.
It is evident from the comparison of mean ∆E
for all the materials and treatments per subgroups of DPI, Protemp™4
and Luxatemp Solar
(Graph 1) that for all the groups, the maximum
color change was seen with the thermocycled
samples stained with sambhar curry solution
and minimum discoloration was seen with
control samples and those stained with coffee.
The intragroup analysis showed that for DPI and
Protemp™4 groups, all the paired comparisons
had statistically significant differences of mean
color stability except comparisons between
control samples vs. samples stained with coffee,
controls vs. samples thermocycled and stained
with coffee and samples stained with coffee vs.
samples thermocycled and stained with coffee
(Table 3, 4). For the Luxatemp Solar group, the only non-significant comparison was between
samples stained with coffee vs. samples
thermocycled and stained with coffee, while rest
were statistically significant. (Table 5).
The intergroup analysis showed that in the
comparison of samples of all experimental
subgroups across different materials (Table 6,
Graph 1), the mean for Luxatemp solar samples
was significantly higher than that of other two
treatment groups, as indicated by P-value <
0.001. In control samples, the mean for Protemp
4 was maximum, followed by DPI and then
Luxatemp solar. The difference in the means,
however, was statistically insignificant.


Perceptible color change of the provisional
material may compromise its acceptability. The
provisional restorative materials chosen were
commonly used ones, with the exception of
Luxatemp Solar dual cure material, a relatively
new material with very limited references in
literature as regards stain resistance.
The spectrophotometer used in this study
provided large area view i.e. a 25.4 mm port
size that had a 22 mm view area for the color
measurement of a sample. Hence, 22 mm
diameter was selected as the size of the samples.
Thickness of 2 mm was selected as it is generally
the maximum facial or occlusal thickness of
a provisional crown and it also allowed ease
of manipulation and polishing.19 Crispin and
Caputo23 found that the color of specimens with
rough surfaces significantly changed. In order
to standardize the procedures, the samples were
finished using coarse pumice as it is routinely
used in clinics for polishing of the restorations.24
The staining solutions, namely coffee solution
and sambhar curry solution were those which are
commonly consumed by the Indian population
and those that have strong potential of staining. Both solutions were prepared in a standardized,
quantifiable manner. The sambhar solution
is a curry that contains most of the Indian
chromatogenic spices that are added in the
food routinely like turmeric powder and red
chili powder.7 It also contains a mixture of many
Indian spices used – coriander, cumin, Bengal
gram, black gram, pigeon pea, fenugreek,
rice, common salt, curry leaf, tamarind, cassia
and asafoetida, all of which have staining
properties. This solution was the representative
liquid of Indian food having multiple spices and
condiments added and it showed the maximum
discoloration in all the materials. Gupta25 stated
that the yellow-orange color of turmeric is due
to a conjugated diarylhepnoid like Curcumin
(3%), which is an active substance, also known
as Natural Yellow. The uptake of this colorant by
the resins causes staining. Munde and Radke7
found that sambhar curry solution has the highest
staining potential, followed by tea solution
and then tobacco solution. The combination
of various strong chromatogens and spices in
sambhar, commonly found in Indian curries,
could be the reason for the highly significant
discoloration produced by this solution.
Guler et al12 has shown that the addition of the
sugar and milk powder in beverages results
in increased color change and the differences
were found to be significant. In the present
investigation, pre weighed, commercially
available sachets were used as it contained
coffee, sugar and milk powder in fixed amounts
for standardisation. The coffee solution showed
less discoloration compared to sambhar curry
solution and the discoloration caused was
clinically acceptable (ΔE < 3.7). Chan, Fuller
and Hormati26 found that coffee caused more
discoloration than tea and cola beverages.
In contrast to these findings, Um and Ruyter27
reported that tea caused more discoloration than
coffee after 48 hours of storage. Absorption and
penetration of colorants into the organic phase
of the resin-based material is probably due to compatibility of the polymer phase with the yellow
colorants of coffee. Smaller molecular size of
coffee coupled with the absorption phenomenon
is said to be the cause of the staining potential
of coffee.20 Coffee also contains large amounts
of staining agent like gallic acid which could be
another reason for its staining capacity.28
The solutions were diluted with artificial saliva
with a ratio of 1:26,17 and stored at 37ºC in a
thermostatically controlled incubator to simulate
intra oral environment.18,19 Samples of the control
group were dipped in commercially available
artificial saliva as provisional restorations
are bathed in saliva in the mouth. This group
also represented the population whose diet
was simple and did not include coffee or
curries. Discoloration produced by this group
was clinically not perceptible (ΔE < 3.7) and
intergroup comparisons were non-significant.
Thermal stress may affect the surface and
structural integrities of resin materials and
render the restorations more susceptible to
staining and discoloration.14 In the present
study, thermocycled samples showed more
discoloration than the non-thermocycled ones
in all the three material groups. The results may
be explained by Strohaver and Mattie 28 who
stated that thermal energy, being sufficiently
capable of causing decomposition of the organic
components present in the resins, leads to the
significant chromatic changes after thermal
cycling. Thermocycling promotes volumetric
contraction and expansion of materials, leading
to degradation. Oliveira et al15 found that
thermocycling increased the surface roughness
in most resins, which may also be the cause of
increased discoloration seen with thermocycled
samples in the present study.
Chemical discoloration has been attributed
to the oxidation of polymer matrix or oxidation
of unreacted double bonds in the residual
monomers and subsequent formation of degradation products from water diffusion.27 In
the present study, methyl methacrylate material
(DPI) was more color stable than the bisacryl
composite materials (Protemp™4 and Luxatemp
Solar). According to Haselton, Diaz-Arnold and
Dawson6, bis-acryl resins showed lesser color
stability as compared to polymethyl methacrylate
(PMMA) since bis-acryl polymers are more polar
than PMMA polymers and therefore have greater
affinity towards water and other polar liquids.
Yannikakis et al30 found that composite based
resins can absorb water at a higher rate because
of the high diffusion coefficient in comparison to
methyl methacrylate. Hence, the present study is
in agreement with these studies.
Strohaver and Mattie 28 showed that the degree of
polymerization is critical to clinical performance
of any resin system and that the degree of
polymerization is not only dependent on type of
resin used, but also the method of polymerization.
They also found fewer voids in heat cure
specimens than in self cured and light cured
specimens. The use of heat for curing produced
a higher degree of polymerization. Khokar et
al31 found that air voids in the resin material
may lead to inhibition zones of unpolymerized
material, resulting in lower color stability. This
may be the reason of the lower color stability of
dual cure (Luxatemp Solar) and chemical cure
(Protemp™4) provisional materials as compared
to the heat cure provisional resin (DPI).
The color stability of the tested provisional
restorative material thus depends on the
chemical composition of that material, the type
of polymerisation and the environment it is
subjected to.
Within the limitations of the study, following conclusions can be drawn: